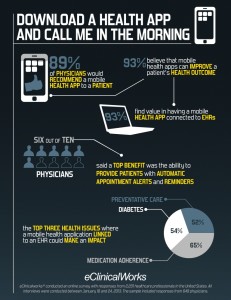
Over one year ago, an eClinicalWorks survey found that 9 in 10 physicians would be interested in prescribing a mobile health app to a patient. That’s a big number. That’s “interest,” but that demand hasn’t yet been expressed in the current go-go app-happy environment.
An opinion piece in this week’s Online First edition of the Journal of the American Medical Association (JAMA) demonstrates the fork-in-the-road facing clinicians and the disruption/opportunity that is mobile health.
In “In Search of a Few Good Apps,” a Boston-based trio of writers (two physicians and one PhD) talk about “the bewildering diversity of apps available [which] has made it difficult for clinicians and the public to discern which apps are the safest or most effective.”
The team says that while the FDA has focused on managing the safety risks of mHealth apps, the Agency has relegated the “review and certification of apps to the marketplace.”
This has resulted in mHealth users – clinicians and consumers – voting with their feet in terms of downloads (where those numbers are transparent), word-of-mouth recommendations, and online reviews (where users are motivated to chime in). But this so-called market-based dynamic isn’t the evidence-based foundation that professionals in medicine need for decision-making with patients at the point-of-care. Nor does the lack of evidence-based reviews help consumers when they seek to DIY their own health (in concert with physicians or on their own).
The authors offer several recommendations to solve this problem of evidence-based discoverability for mHealth apps:
- Organizations can review the quality of the most commonly-used and clinically-useful apps
- Develop guidelines to help developers build quality apps (e.g., safety, accuracy, security, among other criteria); these guidelines could then be criteria on which reviews could be based
- Create certification processes to manage risks for users, ensuring privacy and security á la an Underwriters Laboratories designation (a non-profit organization, but certification could also be undertaken by for-profit groups, the team believes); the Office of the National Coordinator of Health IT (ONC) could play a similar role in shepherding certification as it has done for electronic health records (EHRs). ONC could further support developing mHealth data standards that will enable user-generated data and mHealth apps to interoperate with EHRs.
In summary, the authors believe that the mHealth app industry’s future “looks bright. Soon, physicians may prescribe mHealth apps to patients. Ultimately, the authors say, this will only be viable if people – patients and clinicians alike – trust the apps.
Health Populi’s Hot Points: There are moves afoot in the marketplace to bolster evidence and clinician-crowdsourcing for mobile health apps. In December 2013, IMS Health launched the AppNucleus development platform, along with the AppScript store, to support clinicians and developers in building and sharing reviews about health apps. A team from IMS Health was present at the 2014 HIMSS annual conference in Orlando, where the topic of mobile health (and a growing evidence base) was well-evangelized. The AppNucleus/AppScript team also attended the South-by-Southwest Festival in Austin earlier this month, where I spoke with them about this effort and its goals. IMS Health is all about data (Big, Big Data in health), so this program is a natural extension that builds on the company’s deep mine of health data.
History can offer us lessons. Look back on the business of Happtique (tagline: “your prescription for mobile health”), a project of the Greater New York Hospital Association ventures arm. The project had institutional health care credibility, working with 3 trustworthy partners: the Association of American Medical Colleges (AAMC); CGFNS International, a nursing authority on credentials and licensure; and Intertek, a technology testing and certifying organization.
Happtique announced certification of a first group of mobile health apps in early December 2013, which included CalorieKing, Cardiio, GenieMD, GreatCall, iHealthVentures, MyNetDiary, Osmosis, Power20. QxMD Medical, and Tactio Health Group. Within a week and a half of that announcement, Harold Smith who leads Monkton Health raised concerns about the security testing aspects of Happtique’s certification process in a blog post on his personal site titled “Certification for the lack of certification.” Happtique soon suspended its certification program. (For more on the Happtique outcome, read Brian Dolan’s thorough coverage at MobiHealthNews here published December 13 2013).
A survey conducted on QuantiaMD among 1,500 physicians on the social network, published in February 2014, found that 37% of physicians had already prescribed an app to a patient. But even with that 1 in 3 doctors beginning to recommend apps to patients, QuantiaMD saw that physicians are still guarded about mHealth use:
- 42% won’t prescribe apps because there is no regulatory oversight of them
- 37% have no idea what apps are out there
- 21% never recommend apps to patients
- 21% won’t prescribe apps because there’s no longitudinal data on apps’ effectiveness, and
- 21% won’t prescribe apps because it would generate an overwhelming amount of patient data.
Evidence is crucial to bolster physicians’ faith in mobile apps: “When a prescription drug goes generic, it has at least 7 years of data about its effectiveness and safety, which gives physicians assurance that patients can use it for self-care. Medical apps have no history of either effectiveness or safety,” said Mike Paskavitz, Editor-in-Chief at QuantiaMD.
The JAMA column raises the central issue of trust and evidence, which have different end-points for consumers in a healthcare DIY world versus physicians and nurses whose professional oaths are first, to do no harm. We will continue to Search for a Few Good Apps through our social networks — for patients, in online health social networks in peer-to-peer health care, and via word-of-mouth offline; and for physicians, in communities like Doximity, Ozmosis and QuantiaMD , in practice in clinical organizations, and perhaps through crowdsourcing in the IMS Health AppScript store and similar ventures. Does ONC have the bandwidth to add yet another line-item to its to-do list in a tight-funding environment? With nearly 100,000 mobile health apps available, today’s chaotic mHealth marketplace itself won’t sort this out very soon.




 I'm gobsmackingly happy to see my research cited in a new, landmark book from the National Academy of Medicine on
I'm gobsmackingly happy to see my research cited in a new, landmark book from the National Academy of Medicine on 
 Grateful to Gregg Malkary for inviting me to join his podcast
Grateful to Gregg Malkary for inviting me to join his podcast