 It’s February 1st, which marks the first of 28 days of American Heart Month – a time to get real, embrace, learn about, and engage with heart health.
It’s February 1st, which marks the first of 28 days of American Heart Month – a time to get real, embrace, learn about, and engage with heart health.
Heart disease kills 610,000 people in the U.S. every year, equal to 1 in 4 deaths in America. It’s the leading cause of death for both men and women in the U.S.
Knowing your blood pressure is an important step for managing the risks of heart disease.
That hasn’t yet been available to those of us who quantify our steps, weight, sleep, food intake, and other health metrics. In 2017, Hugh Langley wrote in Wareable that, “blood pressure is wearable tech’s next challenge.”
 I’m glad to report that we’ll be there before the end of the year, based on my meet-up with Omron Healthcare at #CES2018. I spent time with Ranndy Kellogg, CEO, who updated me on the development of the HeartGuide smartwatch which I had previewed last year at CES.
I’m glad to report that we’ll be there before the end of the year, based on my meet-up with Omron Healthcare at #CES2018. I spent time with Ranndy Kellogg, CEO, who updated me on the development of the HeartGuide smartwatch which I had previewed last year at CES.
Omron is submitting the HeartGuide watch for FDA review this year and once cleared, the device will be the first blood pressure monitor that accurately reads BP right from the wrist.
Omron has been one of the few consumer-facing digital health companies that has taken the long-view and done the work to file for FDA clearance for a medical-grade technology that mainstream consumers can use. This places them on a very short list of connected health developers who have patiently developed clinical-grade health techs designed for the consumer market that have undergone regulatory scrutiny.
Once cleared (planned for Fall 2018), the clinical quality of the data output promises to be a huge advance for consumer-marketed wearable technology that doctors can trust. Consumer-purchased activity trackers have been criticized by researchers for a lack of accuracy and consistency. This has prevented them from being embraced and adopted for patients’ use by the medical profession, which has seen a plethora of patients wear activity trackers generating data that has been questioned in peer-reviewed studies.
Omron seeks to jump that hurdle through FDA clearance. Then there’s another critique of many connected health devices which the HeartGuide must overcome: that of usability and accessibility for the consumer. In the case of Omron’s BP watch, it’s the size and weight of typical smartwatch, with legible, easily readable data output on the screen. The device also has a notifications capability similar to other activity tracking watches on the market — but with the clinical, FDA-scrutinized data quality for blood pressure, activity and sleep tracking. Finally, the battery is designed to last for two weeks, which is a consumer-friendly and -desirable feature, as well.
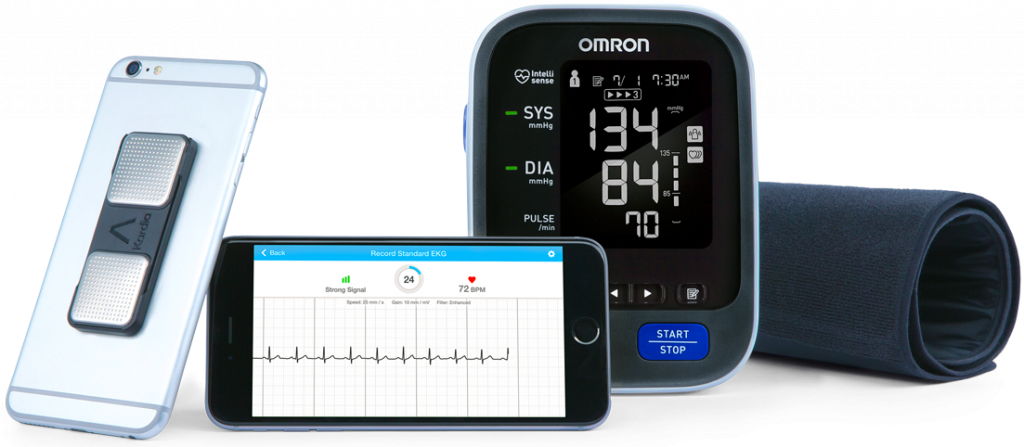 Complementing the HeartGuide, Omron also launched a blood pressure monitor bundled with an EKG device. Stroke accounts for 1 in 20 deaths in the U.S., according to the CDC. Omron’s combined BP and EKG bundle addresses the market of as many as 6 million people who deal with atrial fibrillation (AFib) in the U.S. These patients must be mindful of their risk for stroke: people with AFib have a 5x greater risk of stroke than others. In 2016 at CES, I spoke with Ranndy about the company’s project #GoingforZero, a public health education campaign aiming for “zero” incidence of stroke. I was grateful that this year at Omron’s booth at CES, I could write my name on a Going for Zero poster and as a result, Omron donated a blood pressure monitor to the American Heart Association to use in their public health advocacy program. In this growing era of healthcare collaboration, Omron worked with AliveCor on the BP/EKG technology; here’s more detail on AliveCor’s website here.
Complementing the HeartGuide, Omron also launched a blood pressure monitor bundled with an EKG device. Stroke accounts for 1 in 20 deaths in the U.S., according to the CDC. Omron’s combined BP and EKG bundle addresses the market of as many as 6 million people who deal with atrial fibrillation (AFib) in the U.S. These patients must be mindful of their risk for stroke: people with AFib have a 5x greater risk of stroke than others. In 2016 at CES, I spoke with Ranndy about the company’s project #GoingforZero, a public health education campaign aiming for “zero” incidence of stroke. I was grateful that this year at Omron’s booth at CES, I could write my name on a Going for Zero poster and as a result, Omron donated a blood pressure monitor to the American Heart Association to use in their public health advocacy program. In this growing era of healthcare collaboration, Omron worked with AliveCor on the BP/EKG technology; here’s more detail on AliveCor’s website here.
Voice-tech is growing in healthcare, and Omron has joined the growing list of Alexa skills for consumers managing health-at-home. Rounding out Omron’s #CES2018 announcements is their developing an Alexa skill for people using connected Omron devices that work with the Omron Connect App. Consumers using Omron tech can call o Alexa, open the app, find and hear their recent blood pressure readings, and compare them over time.
Omron’s consumer health products have had a strong market following by older patients, who have traditionally purchased these devices at the retail pharmacy. Being a trusted brand of wearable health tech for older people is a major differentiator for Omron, most importantly because heart disease risks can grow as we age. Increasingly, Omron like other digital health device players, channel products through Amazon, which has a huge connected health store among its growing roster of health-related things (and, potentially, services). [This week, Amazon co-announced a program bringing them together with Warren Buffet’s Berkshire-Hathaway and JP Morgan, to address the high costs of healthcare at the workplace – more on that here in Health Populi].
Here is Omron’s blog written for #CES2018 on Going for Zero.
Health Populi’s Hot Points: In WPP’s Health & Wellness @CES write-up on the event, they observe that we’re moving “from quantifying the self to caring for the self.” They coin this next generation of consumer-facing digital health tech as wearables, carriables, and shareables, supporting consumers managing chronic and pre-existing conditions, and sensing precursors to illness — a category into which the Omron blood pressure watch and EKG bundle also fit.
Physicians are loathe to recommend or prescribe consumer-facing digital health apps and devices that haven’t been proven out with hard data in real people. Cardiac health has a growing evidence base for digital health tech, IQVIA pointed out in their recent study on The Growing Value of Digital Health. After mental health and diabetes, heart apps rank as the third largest categories for medical apps on the market in 2017.
In a 2016 JAMA Cardiology, three medical doctors wrote about the promise of wearable tech devices for preventing and managing heart disease. “As more patients and clinicians become familiar with fitness trackers, these devices could play a larger role in health care….Like mobile phones, these devices may one day become an integral part of society and health care,” they envisioned.
Omron is playing an important role in bridging consumer-facing wearable tech with medical-grade, clinically-approved and prescribable digital health tools that clinicians could embrace into their workflow. More clinicians will welcome these FDA-approved devices in that workflow as they take on more financial risk for patient outcomes and consumer satisfaction for healthcare services.
 Finally, as we are in February, the month to celebrate heart health, here are a few important links highlighting new and exciting developments in the field…
Finally, as we are in February, the month to celebrate heart health, here are a few important links highlighting new and exciting developments in the field…
> More evidence in a large meta-analysis in BMJ on the positive impacts of not smoking and smoking cessation on the risks of coronary heart disease and stroke. [I hasten to connect an important dot today about the resignation of CDC Director Brenda Fitzgerald due to her self-described “complex” financial situation; one aspect of this was her investment in shares in a tobacco company. One of the CDC’s main missions is to educate American health citizens on the health risks of tobacco consumption, and to reduce peoples’ use of tobacco].
> Discovery of a bone-deep risk for heart disease called CHIP (clonal hematopoiesis of indeterminate potential), discussed in great detail in the New York Times. This may be as high a risk as high cholesterol and elevated blood pressure.
> More open discussion about the risk of prediabetes on heart disease, this from the CDC.
> And, for those of you who are mobile payments fans, Samsung filed a patent for a biometric way to authenticate your identity via your blood pressure reading without a PIN or password.





 Thanks to Feedspot for naming this blog, Health Populi, as a
Thanks to Feedspot for naming this blog, Health Populi, as a